
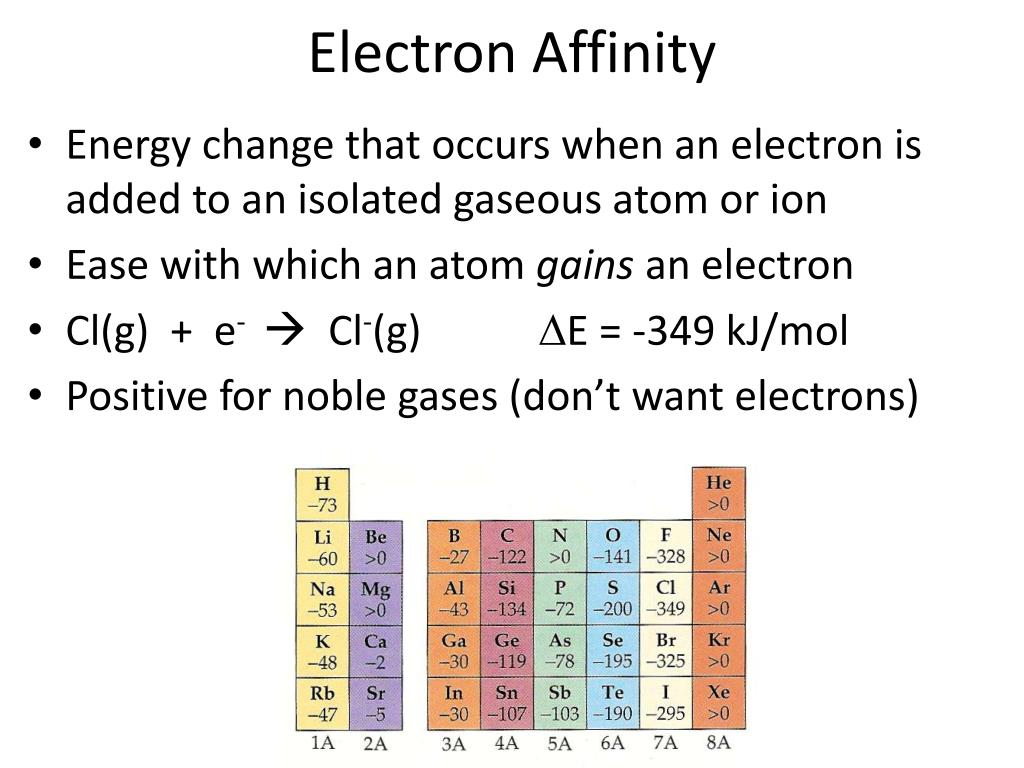
The relation between the two is E ea = −Δ E(attach). Confusion arises in mistaking E ea for a change in energy, Δ E, in which case the positive values listed in tables would be for an endo- not exo-thermic process. It is the word "released" within the definition "energy released" that supplies the negative sign to Δ E. The positive values that are listed in tables of E ea are amounts or magnitudes. Electron capture for almost all non- noble gas atoms involves the release of energy and thus are exothermic. For any reaction that releases energy, the change Δ E in total energy has a negative value and the reaction is called an exothermic process. To use electron affinities properly, it is essential to keep track of sign.
Together they may undergo charge-transfer reactions. Another example, a molecule or atom that has a more positive value of electron affinity than another is often called an electron acceptor and the less positive an electron donor. Other theoretical concepts that use electron affinity include electronic chemical potential and chemical hardness. Mulliken to develop an electronegativity scale for atoms, equal to the average of the electronsĪffinity and ionization potential. This property is used to measure atoms and molecules in the gaseous state only, since in a solid or liquid state their energy levels would be changed by contact with other atoms or molecules.Ī list of the electron affinities was used by Robert S. Measurement and use of electron affinity 4 "Electron affinity" as defined in solid state physics.

1 Measurement and use of electron affinity.


 0 kommentar(er)
0 kommentar(er)
